- Coronary Stents Coronary Balloons Catheters Guide Wires Introducer Sheaths Accessories Peripheral Interventional Products
- Immunoassay Molecular Diagnostics Hematology Diagnostics POCT Lab Solution
- Structural Heart Diseases Products
- Vascular Access Blood Pressure Transducer Arterial Blood Sampler CVC Accessories IV Catheter Needle-free Connectors Insulin Injection Pain Relief
- Hemodialysis consumables Hemoperfutor Dialysis machine
- ECG Patient Monitor Oximeter
- Cardiovasculars Oncology Anti-infectives Central Nervous System Others
- Surgical Staplers Endoscopy Endoscopic Ultrasound System
- Trauma Implant Spinal Implant Artificial Joint Sports Medicine
- Pacemakers and Leads Equipments
- ELISA CLIA FIA
- Nucleic Acid Extraction Nucleic Acid Extraction & PCR Kits Real-Time PCR
- Thrombelastography Analyzer Blood Grouping Platelet Aggregation
- POCT - FIA Colloidal Gold Blood Glucose Cholesterol SARS-CoV-2
- NeoECG Holter Monitor Portable ECG Monitor AI-ECG Pocket ECG
- Patient Monitor Vital Signs Monitor End-tidal Capnography
- Fingertip Oximeter Handheld Oximeter Wrist Oximeter Wearable Oximeter All-in-One Health Monitor POCT Solution
- OBS Locking Compression Plate Locking Plates Conventional Plates Mini Plates System Screws Patella Ring - Patented Product Metal Pins Cable System
- Internal Fixation System Laminoplasty Plate System Percutaneous Kyphoplasty Fusion Cage Cervical Fixation System
- Hip System Knee System
- Suture Anchor Suture Button
SARS-CoV-2 Nucleic Acid Test Kit (PCR-Fluorescence Probe Method)


SARS-CoV-2 Nucleic Acid Test Kit (PCR-Fluorescence Probe Method) Details
Product Description
SARS-CoV-2 Nucleic Acid Test Kit is a real-time PCR test intended for the qualitative detection in ORF1ab and N genes of SARS-CoV-2 viral nucleic acids in pharyngeal specimens (oropharyngeal swabs, nasopharyngeal swabs) from individuals who are suspected of COVID-19 by their healthcare provider.
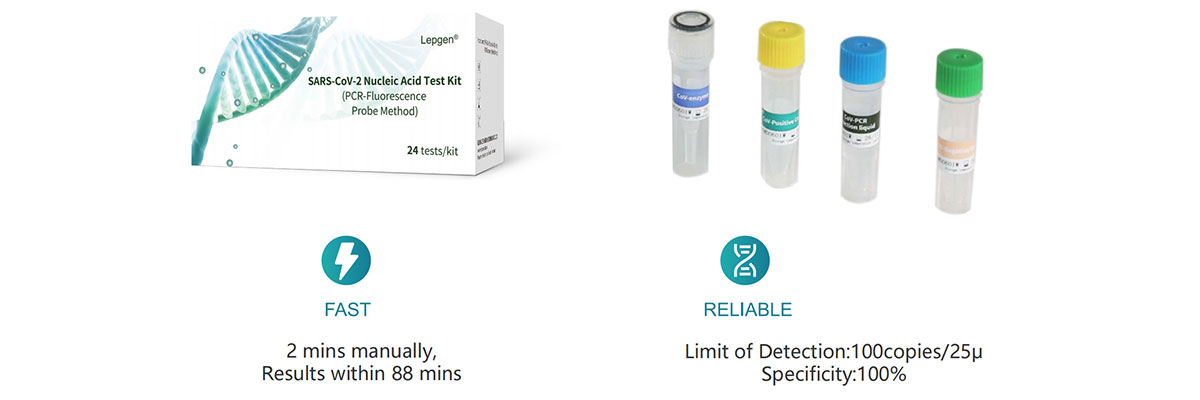
Work Flow
Step 1 Nucleic Acid Extraction
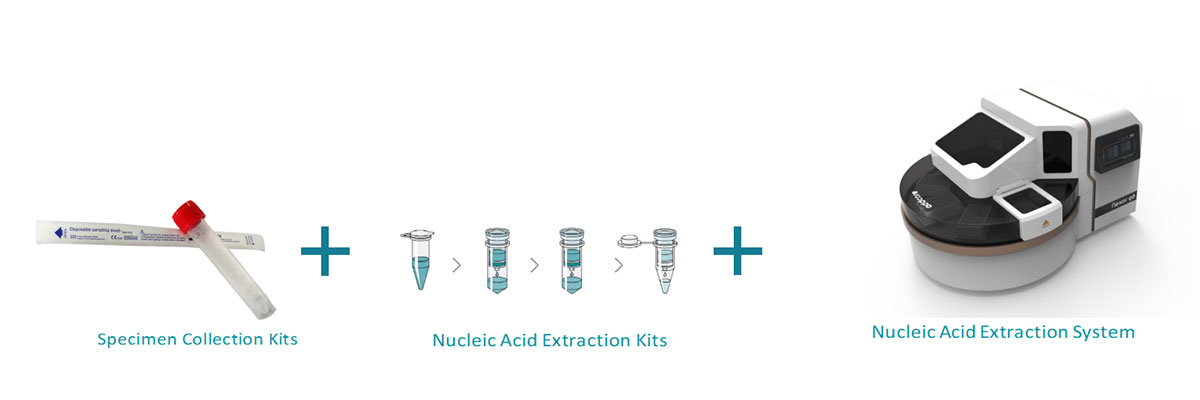
Step 2 RT-PCR Amplification and Analysis

Standard Configurations
| Number of Test / Kit | 24 tests |
| Sample Type | Oropharyngeal swabs, Nasopharyngeal swabs |
| Acceptable Real-time PCR System | LEPU Lepgen-96, Roche LightCycler 480 series Bio-Rad CFX96 |
| Reagent Preparation Condition | 4 weeks at 2℃ ~8℃ |
| Reagent Storage Condition | 6 months at -25℃ ~ -15℃, The freeze/thaw cycles should not exceed five times |
Key Components
Component | Qty. | Volume | Ingredients |
Cov-PCR Reaction Liquid | 1 | 460μl/vial | ORF1ab and N genes primer and probe, Internal control RNase P gene primer and probe, Deoxyribonucleoside triphosphate |
Cov-enzyme | 1 | 24μl/vial | DNA polymerase, Reverse transcriptase, Recombinant RNase inhibitor. |
Cov-Negative Control | 1 | 420μl/vial | DEPC treated water |
Cov-Positive Control | 1 | 420μl/vial | ORF1ab and N genes, RNase P gene armored RNA solution |
Thank You for Your Attention on Lepu Medical!
Email us with any questions or inquiries or use our contact data. We would be happy to answer your questions.
