- Coronary Stents Coronary Balloons Catheters Guide Wires Introducer Sheaths Accessories Peripheral Interventional Products
- Immunoassay Molecular Diagnostics Hematology Diagnostics POCT Lab Solution
- Structural Heart Diseases Products
- Vascular Access Blood Pressure Transducer Arterial Blood Sampler CVC Accessories IV Catheter Needle-free Connectors Insulin Injection Pain Relief
- Hemodialysis consumables Hemoperfutor Dialysis machine
- ECG Patient Monitor Oximeter
- Cardiovasculars Oncology Anti-infectives Central Nervous System Others
- Surgical Staplers Endoscopy Endoscopic Ultrasound System
- Trauma Implant Spinal Implant Artificial Joint Sports Medicine
- Pacemakers and Leads Equipments
- ELISA CLIA FIA
- Nucleic Acid Extraction Nucleic Acid Extraction & PCR Kits Real-Time PCR
- Thrombelastography Analyzer Blood Grouping Platelet Aggregation
- POCT - FIA Colloidal Gold Blood Glucose Cholesterol SARS-CoV-2
- NeoECG Holter Monitor Portable ECG Monitor AI-ECG Pocket ECG
- Patient Monitor Vital Signs Monitor End-tidal Capnography
- Fingertip Oximeter Handheld Oximeter Wrist Oximeter Wearable Oximeter All-in-One Health Monitor POCT Solution
- OBS Locking Compression Plate Locking Plates Conventional Plates Mini Plates System Screws Patella Ring - Patented Product Metal Pins Cable System
- Internal Fixation System Laminoplasty Plate System Percutaneous Kyphoplasty Fusion Cage Cervical Fixation System
- Hip System Knee System
- Suture Anchor Suture Button

2019-nCoV Neutralization Antibody Test Kit (ELISA) Details
Product Description
2019-nCoV Neutralization Antibody Test Kit (ELISA) is a rapid test used for detecting or quantifying antibody (Ab) against viruses, bacteria and other materials or antigen (Ag). ELISA is so named because the test technique involves the use of an enzyme system and immunosorbent.
Product Features
• High sensitivity (98.61%)
• High specificity (99.11%)
• Rapid results
Method:
Enzyme-linked Immunosorbent Assay.
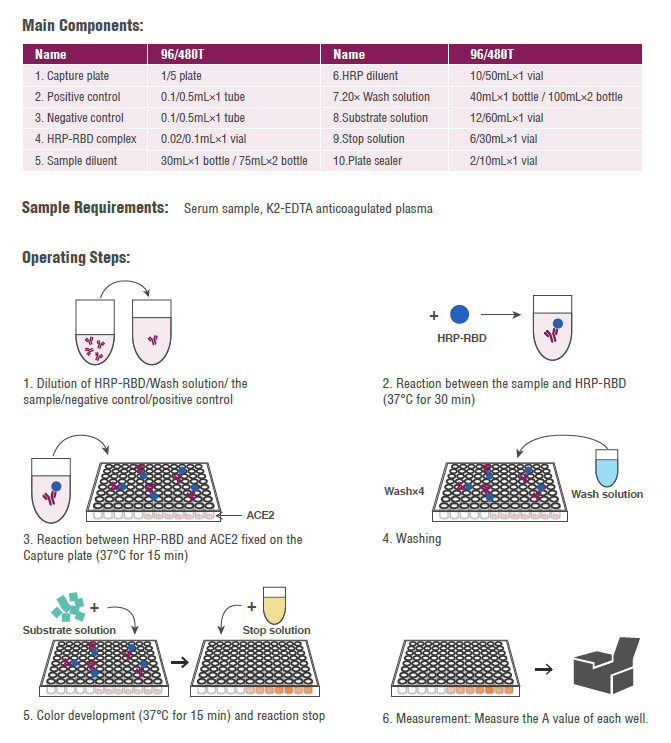
Result Interpretation:
• The negative/positive cutoff value of 2019-nCoV neutralizing antibody is judged by the inhibition rate. Inhibition rate = (1 - sample OD value/ negative quality control OD value) × 100%
• Inhibition rate ≥ 20% is positive, which means that the 2019-nCoV neutralizing antibody is detected;
• Inhibition rate<20 is negative, which means that the 2019-nCoV neutralizing antibody is not detected.
Thank You for Your Attention on Lepu Medical!
Email us with any questions or inquiries or use our contact data. We would be happy to answer your questions.
