Products
Cardiovascular & Peripheral Products
In Vitro Diagnosis
Structural Heart Diseases Products
Critical Care Products
Hemodialysis Products
ECG & Patient Monitor & Telehealth Solutions
Pharmaceutical (API) Products
Surgical Products
Orthopedic Products
Cardiac Rhythm Management Products
Skin Care
Digital Subtraction Angiography Systems
- Coronary Stents Coronary Balloons Catheters Guide Wires Introducer Sheaths Accessories Peripheral Interventional Products
- Immunoassay Molecular Diagnostics Hematology Diagnostics POCT Lab Solution
- Structural Heart Diseases Products
- Vascular Access Blood Pressure Transducer Arterial Blood Sampler CVC Accessories IV Catheter Needle-free Connectors Insulin Injection Pain Relief
- Hemodialysis consumables Hemoperfutor Dialysis machine
- ECG Patient Monitor Oximeter
- Cardiovasculars Oncology Anti-infectives Central Nervous System Others
- Surgical Staplers Endoscopy Endoscopic Ultrasound System
- Trauma Implant Spinal Implant Artificial Joint Sports Medicine
- Pacemakers and Leads Equipments
- ELISA CLIA FIA
- Nucleic Acid Extraction Nucleic Acid Extraction & PCR Kits Real-Time PCR
- Thrombelastography Analyzer Blood Grouping Platelet Aggregation
- POCT - FIA Colloidal Gold Blood Glucose Cholesterol SARS-CoV-2
- NeoECG Holter Monitor Portable ECG Monitor AI-ECG Pocket ECG
- Patient Monitor Vital Signs Monitor End-tidal Capnography
- Fingertip Oximeter Handheld Oximeter Wrist Oximeter Wearable Oximeter All-in-One Health Monitor POCT Solution
- OBS Locking Compression Plate Locking Plates Conventional Plates Mini Plates System Screws Patella Ring - Patented Product Metal Pins Cable System
- Fusion Cage Cervical Fixation System Internal Fixation System Laminoplasty Plate System Percutaneous Kyphoplasty
- Hip System Knee System
- Suture Anchor Suture Button

Disposable Postoperative Pain Relief System
Directly block the pain at the surgical siteReduce the useof opioidsImprove the comfort of anesthesia and analgesia for patients


Disposable Postoperative Pain Relief System Details
Product Description
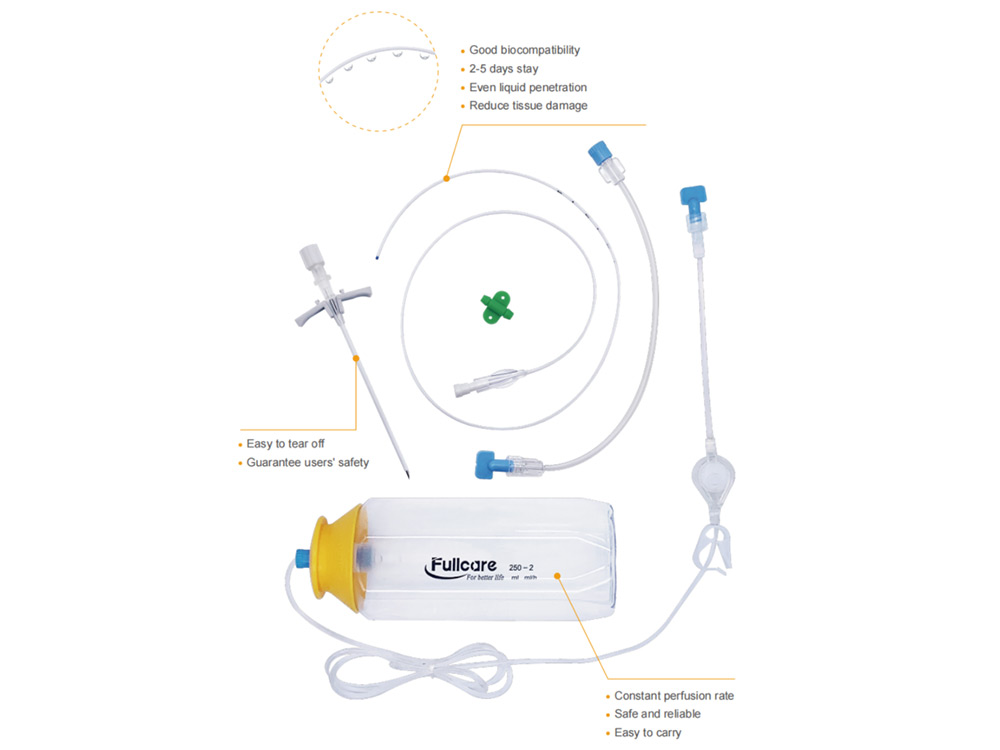
Specifications of Disposable Postoperative Pain Relief System
| Model | Length of infusion catheter(mm) | Number of Catheter (pcs) | Capacity of Infusion pump(ml) | Flow rate(ml/h) |
| TJPS040-1-100-2 | 40 | 1 | 100 | 2 |
| TJPS060-1-100-2 | 60 | 1 | 100 | 2 |
| TJPS060-1-250-2 | 60 | 1 | 250 | 2 |
| TJPS060-1-250-5 | 60 | 1 | 250 | 5 |
| TJPS060-2-250-5 | 60 | 2 | 250 | 5 |
| TJPS080-1-100-2 | 80 | 1 | 100 | 2 |
| TJPS080-1-250-2 | 80 | 1 | 250 | 2 |
| TJPS080-1-250-5 | 80 | 1 | 250 | 5 |
| TJPS080-2-250-5 | 80 | 2 | 250 | 5 |
| TJPS100-1-100-2 | 100 | 1 | 100 | 2 |
| TJPS100-1-250-2 | 100 | 1 | 250 | 2 |
| TJPS100-1-250-5 | 100 | 1 | 250 | 5 |
| TJPS100-2-250-5 | 100 | 2 | 250 | 5 |
| TJPS120-1-100-2 | 120 | 1 | 100 | 2 |
| TJPS120-1-250-2 | 120 | 1 | 250 | 2 |
| TJPS120-1-250-5 | 120 | 1 | 250 | 5 |
| TJPS120-2-250-5 | 120 | 2 | 250 | 5 |
| TJPS040-3-250-5 | 40 | 3 | 250 | 5 |
| TJPS060-3-250-5 | 60 | 3 | 250 | 5 |
| TJPS-2-250-5-120x040 | 12040 | 2 | 250 | 5 |
| TJPS-2-250-5-120x060 | 12060 | 2 | 250 | 5 |
| TJPS-2-250-5-100x060 | 10060 | 2 | 250 | 5 |
| TJPS-2-250-5-100x040 | 10040 | 2 | 250 | 5 |
| TJPS-2-220-3.5-120x040 | 12040 | 2 | 220 | 3.5 |
| TJPS-2-220-3.5-120x060 | 12060 | 2 | 220 | 3.5 |
| TJPS-2-220-3.5-060x060 | 6060 | 2 | 220 | 3.5 |
| TJPS-2-220-3.5-080x080 | 8080 | 2 | 220 | 3.5 |
| TJPS-2-220-3.5-100x100 | 100100 | 2 | 220 | 3.5 |
| TJPS-2-220-3.5-120x120 | 120120 | 2 | 220 | 3.5 |
| TJPS-3-250-5-100x100x040 | 10010040 | 3 | 250 | 5 |
| TJPS-3-250-5-100x100x060 | 10010060 | 3 | 250 | 5 |
| TJPS-3-250-5-120x120x060 | 12012060 | 3 | 250 | 5 |
| TJPS-3-250-5-120x120x040 | 12012040 | 3 | 250 | 5 |
Clinical Trial Reference
This product has been randomly tested in 160 cases in Peking University Hospital and the Third Hospital of Peking University. The test results are as follows:
| Statistics table | Control group (intravenous morphine PCA, number of cases and percentage) | Test group (Continuous local analgesia, lidocaine, number and percentage of cases) | Statistics Volume | P Value | Conclusion | |
| Pain 24 hrs post-surgical | Use of auxiliary analgesics | 10(12.50) | 14(17.50) | 0.784a | 0.376a | No significant difference |
| Pain level | 0 N(%) | 32(40.00) | 27(33.75) | 0.741b | 0.459b | No significant difference |
| 1 N(%) | 26(32.50) | 28(35.00) | ||||
| 2 N(%) | 21(26.25) | 25(31.25) | ||||
| 3 N(%) | 1(1.25) | 0(0.00) | ||||
| Pain 48 hrs post-surgical | Use of auxiliary analgesics | 10(12.50) | 17(21.25) | 0.784a | 0.376a | No significant difference |
| Pain level | 0 N(%) | 36(45.00) | 36(45.00) | 0.173b | 0.863b | No significant difference |
| 1 N(%) | 42(52.50) | 44(55.00) | ||||
| 2 N(%) | 2(2.50) | 0(0.00) | ||||
| 3 N(%) | 0(0.00) | 0(0.00) | ||||
| Patient satisfaction with anesthetic infusion device | Satisfied | 76(95.00) | 78(97.50) | 0.681* | No significant difference | |
| Unsatisfied | 4(5.00) | 2(2.50) | ||||
| Safety analysis | Side effects(urinary retention,nausea and vomiting,etc.) | 7 | 3 | 1.7067 | 0.191 | No significant difference |
Thank You for Your Attention on Lepu Medical!
Email us with any questions or inquiries or use our contact data. We would be happy to answer your questions.
