- Coronary Stents Coronary Balloons Catheters Guide Wires Introducer Sheaths Accessories Peripheral Interventional Products
- Immunoassay Molecular Diagnostics Hematology Diagnostics POCT Lab Solution
- Structural Heart Diseases Products
- Vascular Access Blood Pressure Transducer Arterial Blood Sampler CVC Accessories IV Catheter Needle-free Connectors Insulin Injection Pain Relief
- Hemodialysis consumables Hemoperfutor Dialysis machine
- ECG Patient Monitor Oximeter
- Cardiovasculars Oncology Anti-infectives Central Nervous System Others
- Surgical Staplers Endoscopy Endoscopic Ultrasound System
- Trauma Implant Spinal Implant Artificial Joint Sports Medicine
- Pacemakers and Leads Equipments
- ELISA CLIA FIA
- Nucleic Acid Extraction Nucleic Acid Extraction & PCR Kits Real-Time PCR
- Thrombelastography Analyzer Blood Grouping Platelet Aggregation
- POCT - FIA Colloidal Gold Blood Glucose Cholesterol SARS-CoV-2
- NeoECG Holter Monitor Portable ECG Monitor AI-ECG Pocket ECG
- Patient Monitor Vital Signs Monitor End-tidal Capnography
- Fingertip Oximeter Handheld Oximeter Wrist Oximeter Wearable Oximeter All-in-One Health Monitor POCT Solution
- OBS Locking Compression Plate Locking Plates Conventional Plates Mini Plates System Screws Patella Ring - Patented Product Metal Pins Cable System
- Fusion Cage Cervical Fixation System Internal Fixation System Laminoplasty Plate System Percutaneous Kyphoplasty
- Hip System Knee System
- Suture Anchor Suture Button

MemoCarna® Atrial Septal Defect (ASD) Occluder
Reduce the amount of metal implantation
Easy to release and recycle
Umbrella surface is flatter
Less nickel ions precipitated
Excellent push performance


Product Description
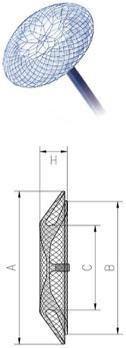
MemoCarna® Atrial Septal Defect (ASD) Occluder with single hub
The product is composed of a nickel titanium alloy stent, a stainless steel bushing and a polyester fiber membrane.
The stent is made of medical nickel-titanium memory alloy wires and filled with the polyester fiber membrane, to be applied for the atrial septal defect.
A stainless steel screw bushing used for fixing the nickel-titanium alloy wire on one end thereof, and the nut of the steel screw bushing may match with the screw of the head end of the conveyor pushing rod.
Product Features
Atrial Septal Defect Occluder adopts unique Dature-shape braiding technology, achieves faster endothelization with more flat disk after releasing and decreasing influences to blood flow.
Atrial Septal Defect Occluder updated surface treatment technology, forming uniform and even oxide layer while removing impurities. Nickle ions release is effectively reduced and delivery performance is enhanced.
Indications for Use
Suitablefor the secundum left-to-right shunt atrial septal defect (ASD) with the ASD diameter of ≥ 5mm and ≤ 36mm and the increased right ventricular volume overload;
The defect edge is ≥ 5mm distanced from the coronary sinus, the superior or inferior vena cava and the pulmonary vein, and is ≥ 7mm distanced from atrioventricular valve.
Other cardiac abnormalities required for the surgery are not combined.
Product Highlights
Well coordinated with delivery system, multi withdraw, release and antifatigue performance.
After 3 months implantation, CARNA occluder positioned well, without residual shunt, smooth two disks surface without thrombus or neoplasms.
Product Advantages
Adopt updated structure braiding technology, with advanced heat treatment technique,
guarantee additional advantages:
Effectively decreased nitinol ions release into human body.
Accelerated endothelialization process, minimize thrombus risk.
Single hub design reduce metal implantation and device-driven thrombus risk.
Ordering Information
| Catalogue No | C Waist Diameter (mm) | H Height (mm) | A Left Disc Diameter (mm) | B Right Disc Diameter (mm) |
| DMFQFDQ-104 | 4.0±0.5 | 5.5±1.0 | 18.0±1.0 | 14.0±1.0 |
| DMFQFDQ-105 | 5.0±0.5 | 5.5±1.0 | 19.0±1.0 | 15.0±1.0 |
| DMFQFDQ-106 | 6.0±0.5 | 5.5±1.0 | 20.0±1.0 | 16.0±1.0 |
| DMFQFDQ-107 | 7.0±0.5 | 5.5±1.0 | 21.0±1.0 | 17.0±1.0 |
| DMFQFDQ-108 | 8.0±0.5 | 5.5±1.0 | 22.0±1.0 | 18.0±1.0 |
| DMFQFDQ-109 | 9.0±0.5 | 5.5±1.0 | 23.0±1.0 | 19.0±1.0 |
| DMFQFDQ-110 | 10.0±0.5 | 5.5±1.0 | 24.0±1.0 | 20.0±1.0 |
| DMFQFDQ-111 | 11.0±0.5 | 5.5±1.0 | 25.0±1.3 | 21.0±1.3 |
| DMFQFDQ-112 | 12.0±0.5 | 5.5±1.0 | 26.0±1.3 | 22.0±1.3 |
| DMFQFDQ-113 | 13.0±0.5 | 5.5±1.0 | 27.0±1.3 | 23.0±1.3 |
| DMFQFDQ-114 | 14.0±0.5 | 5.5±1.0 | 28.0±1.3 | 24.0±1.3 |
| DMFQFDQ-114 | 15.0±0.5 | 5.5±1.0 | 29.0±1.3 | 25.0±1.3 |
| DMFQFDQ-116 | 16.0±0.5 | 5.5±1.0 | 30.0±1.5 | 26.0±1.5 |
| DMFQFDQ-117 | 17.0±0.5 | 5.5±1.0 | 31.0±1.5 | 27.0±1.5 |
| DMFQFDQ-118 | 18.0±0.5 | 5.5±1.0 | 32.0±1.5 | 28.0±1.5 |
| DMFQFDQ-119 | 19.0±0.5 | 5.5±1.0 | 33.0±1.5 | 29.0±1.5 |
| DMFQFDQ-120 | 20.0±1.0 | 5.5±1.0 | 34.0±1.5 | 30.0±1.5 |
| DMFQFDQ-122 | 22.0±1.0 | 5.5±1.0 | 36.0±1.5 | 32.0±1.5 |
| DMFQFDQ-124 | 24.0±1.0 | 6.0±1.0 | 38.0±1.5 | 34.0±1.5 |
| DMFQFDQ-126 | 26.0±1.0 | 6.0±1.0 | 40.0±1.5 | 36.0±1.5 |
| DMFQFDQ-128 | 28.0±1.0 | 6.0±1.0 | 42.0±1.5 | 38.0±1.5 |
| DMFQFDQ-130 | 30.0±1.0 | 6.0±1.0 | 44.0±1.5 | 40.0±1.5 |
| DMFQFDQ-132 | 32.0±1.0 | 6.0±1.0 | 46.0±1.5 | 42.0±1.5 |
| DMFQFDQ-134 | 34.0±1.0 | 6.0±1.0 | 48.0±1.5 | 44.0±1.5 |
| DMFQFDQ-136 | 36.0±1.0 | 6.0±1.0 | 52.0±1.5 | 46.0±1.5 |
| DMFQFDQ-138 | 38.0±1.0 | 6.0±1.0 | 54.0±1.5 | 50.0±1.5 |
| DMFQFDQ-140 | 40.0±1.0 | 6.0±1.0 | 56.0±1.5 | 52.0±1.5 |
Thank You for Your Attention on Lepu Medical!
Email us with any questions or inquiries or use our contact data. We would be happy to answer your questions.
